ZSR Vortices offers assistance in training, inspection, consultancy, auditing and certification services in :
• Quality Management Systems - ISO 9001 (QMS)
• Environmental Management Systems - ISO14001 (EMS)
• Occupational Health & Safety - ISO 45001 (OHS)
• Occupational Health & Safety - OHSAS 18001 (OHSMS)
• QMS for Medical Device Industry - ISO 13485
• Good Distribution Practice For Medical Devices (GDPMD)
• QMS for Automotive Industry - ISO/TS 16949 & VDA (German Automotive Industry Standard)
• Information Security Management System – ISO 27001 (ISMS)
• QMS for Oil & Gas Industry - ISO/TS 29001 and API SPEC. Q1
• Integrated Management System - IMS
• Good Manufacturing Practice, Good Hygiene Practice, - GMP/GHP
• Food Safety Program - ISO 22000 / Hazard Analysis Critical Control Point (HACCP)
• Competency of testing and calibration laboratories - ISO 17025:2005
ISO MANAGEMENT SYSTEMS - TRAINING & AUDIT SERVICES
ISO 9001
Expectations of Customers are growing and they are becoming more sophisticated and better informed. For any business, the only way to keep up is to offer a commitment to quality. In fact any organization, whatever their size or type of industry, they can give themselves a secure future by introducing a quality management system (QMS) such as the ISO 9001.
Benefits of implementing a quality management system (QMS) include:

• "Top Management” set the policies and objectives
• understanding the customer's requirements in order to achieve customer satisfaction
• improved internal and external communications
• better understanding of the organization's processes
• reduced wastage
• responsibilities and authorities are clearly defined and agreed by all staff
• understanding how statutory and regulatory requirements impact the organization and its customers
• improved use of time and resources such as manpower, equipment & material
• greater consistency and traceability of products and services
• improved morale and motivation of personnel
A quality management system in accordance with ISO 9001 will provide the organization with a set of processes that ensure a common sense approach to the management of the organisation.
The system should ensure consistency and improvement of working practices, which in turn should provide products and services that meet customer's requirements. ISO 9001 is the most commonly used international standard that provides a framework for an effective quality management system.
Note: The International Organization for Standardization (ISO) is scheduled to release the revised standard (ISO 9001:2015) in September, 2015. As such, we are offering transition training, audit and consultancy services to the new requirements based on the ISO/DIS 9001:2015. A copy of the ISO/DIS 9001:2015 is available on our Downloads page for your training purposes.
EMS (ISO 14001).
A company's environmental performance can have a significant impact on its success. ISO 14001 is the internationally recognized standard for Environmental Management Systems (EMS). An Environmental Management System provides a framework for managing environmental responsibilities so they become more efficient and more integrated into overall business operations. Environmental Management Systems are based on standards, which specify a process of achieving continuously improved environmental performance and compliance with legislation.
ZSR VORTICES offers assistance for ISO 14001 certifications which will help the Organisation achieve the requirements of this standard and so enhance the organisation's competitive advantage and
reputation.
. 

Certification can :
• Demonstrate high environmental standards
• Demonstrate compliance with legislation
• Reduce costs
• Improve overall efficiency and performance
• Remove uncertainty and inconsistency by managing disruption and waste
• Give competitive advantage to avoid international trade barriers
Note: The International Organization for Standardization (ISO) has revised the ISO 9001 (Quality), ISO 14001 (Environment) in 2015 and ISO 45001 (Safety) in 2018 respectively. We are offering training, audit and consultancy services on the new requirements for these Standards.
ISO 45001 OR OHSAS 18001
Health and safety at work is an issue affecting all businesses. Implementing an occupational health and safety (OHS) management system is now a legal requirement in many countries. How do you keep up with legislation and set up a system that protects the staff and the Organisation? The answer is to introduce a management system that can reduce the risk of accidents, litigation and downtime.
An Occupational Health and Safety Management System provides a framework for managing OHS responsibilities so they become more efficient and more integrated into overall business operations. OHS Management systems are based on standards, which specify a process of achieving continuously improved OHS performance and complying with legislation. ZSR VORTICES offers assistance for certification to the internationally recognized assessment specification, OHSAS 18001 (which was based on the original British Standard BS 8800). ISO 45001:3018 is recognised in more than 160 counties. Its structure is inline with ISO 9001:2015 (QMS) and ISO 14001:2015 (EMS) respectively.
Certification can:

• Reduce risk of accidents, litigation and downtime
• Give competitive advantages
• Help companies stay in compliance with legal requirements
• Improve overall performance
HAZARD ANALYSIS CRITICAL CONTROL POINT (HACCP)
Hazard Analysis Critical Control Points (HACCP) is the main platform for international legislation and good manufacturing practices for all sectors of the food industry. HACCP also forms a key component of many certified compliance standards and is recognized as a main element of international trade in food products. HACCP is a risk management tool recognized internationally for use in the proactive management of food safety issues. A HACCP system helps you to focus on the hazards that affect food safety through hazard identification and to establish critical control limits at critical points during the production process.
The Codex Alimentarius Commission (CAC) has developed international codex standards and guidelines with the aim of providing a high level of consumer protection and fair practice in the international trade of food and agricultural products.

HACCP is relevant to all sectors of the food industry, including primary producers, manufacturers, processors and food service operators who want to demonstrate their compliance with national or international food safety legislation requirements.
There are clear benefits associated with HACCP-based adoption and certification:
• Enables the Organisation to demonstrate a commitment to food safety
• Conveys a degree of confidence required by consumers, retailers and buyers within the food industry
• Provides buyers, consumers, government enforcement and trade agencies with justified assurance that control systems are in place to assure the safe production of food
• It is based on the internationally-recognized Codex Alimentarius standards and guidelines and other national standards
• Regular assessments help the Organisation to continually monitor its food safety system.
GOOD DISTRIBUTION PRACTICE FOR MEDICAL DEVICES (GDPMD)
Introduction
The involvement of various organizations for the handling, transportation, storage, distribution and tracking of medical devices may pose risks to the products. The risks could be from the mix-ups, contamination as well as the counterfeit and tampered products.
Therefore, appropriate management and control shall be applied over these activities to ensure the maintenance of the quality and the integrity of the medical devices throughout the distribution process.
GDPMD specifies the requirements for a quality system to be established and maintained by an organization in carrying out activities in the medical devises supply-chain. GDPMD requires an organization to demonstrate its ability to maintain safety and performance of medical devices throughout the supply-chain. It will be used by both internal and external parties to determine the ability of an establishment to meet the requirements specified within.
Ensuring delivery of safe products shall be the priority of the parties involved in the distribution of the medical devices. The industry and the public, including the users are at the risks of having counterfeit, mix-ups, contamination and cross contamination products.
In order to address these perennial risks, the medical devices distribution industry shall work in tandem with the manufacturers as soon as the products leave the factories. Therefore, pertinent good distribution practices shall be applied to all organizations responsible for the warehousing and distributing medical devices in the supply chain.
Background Information

Medical Device Act 2012 (Act 737)
- has been gazetted on 9th February, 2012
- will come into effect on 1st July 2013 and undergoes a transition period before it is fully enforced in two years’ time.
- specifies requirements for medical device product registration, establishment licensing & conformity assessment body (CAB) registration.
Medical Device Authority Act 2012 (Act 738)
- has been gazetted on 9th February, 2012
- The Act details out the organisation of a regulatory body that will implementing Act 737.
Medical Device Regulations 2012
- the subsidiary legislations under the Medical Device Act 2012 (Act 737)
- has been approved by the Minister of Health (MOH) and has been published in the Gazette on 31st December, 2012.
- specifies requirements and procedural matters pertaining to medical device registration, conformity assessment body (CAB) registration, establishment licensing, export permit and appeal.
- will come into operation simultaneously with Act 737 on 1st July 2013. And as specified in Act 737 a transition period of two years for medical device registration and one year for establishment licensing will be given to the industry before it is fully enforced.
FOOD SAFETY MANAGEMENT SYSTEM (ISO 22000)
Safe food is critical for consumers and businesses.

While much of our food supply is safe, several recent high profile cases around the world underline the potential danger of food-borne illness to consumers, employees and brand value. A few recent examples include BSE infected beef, the salmonella contamination of poultry and eggs and high levels of lister in dairy products. For these reasons and others, global retailers, distributors, food manufacturers and food service companies are now concerned more about the safety of their food supply chain than ever before.
Organisations in the food sector will need to manage risk, demonstrate good corporate responsibility and meet legal requirements if they are to remain competitive, protect their reputation and enhance their brand. An effective food safety management system based on a proven standard will help the organisation achieve these goals. Furthermore, assessment and certification of its management system by an independent accredited third party ISO Certification Body will optimize its food safety management.
QMS IN MEDICAL DEVICES (ISO 13485)
ISO 13485, published in 2003, and now fully recognized in many countries, is based on the ISO 9001 process model approach. These standards provide a good base model, recognised by the Global Harmonization Task Force (GHTF), for compliance with the European Union (EU) CE Marking medical device directives, Health Canada CMDCAS, Taiwan Medical Device Regulations, Japan JPAL and other international requirements.

Certification to ISO 13485 takes place when an accredited third party, such as ISO Certification Body, visits an organization, assesses the quality management system and, if satisfactory, issues a certificate confirming that the organization’s quality management system meets the requirements of this standard.
ISO 13485 quality management systems certificate is an objective, accredited, recognized evidence of the Organisation’s commitment to quality systems and helps it demonstrate this to its customers and regulators. ISO 13485 is becoming widely accepted as the international standard to address medical device requirements around the world.
The standard can be used by manufacturers to demonstrate compliance to applicable regulatory requirements, and by other organisations whose related services support medical device manufacturers.
ISO 27001 (INFORMATION SECURITY MANAGEMENT SYSTEM)
ISO 27001 establishes guidelines and general principles for initiating, implementing, maintaining, and improving information security management in an organization. The objectives outlined provide general guidance on the commonly accepted goals of information security management.
ISO 27001 contains best practices of control objectives and controls in the following areas of information security management:
• security policy;
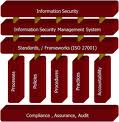
• organization of information security;
• asset management;
• human resources security;
• physical and environmental security;
• communications and operations management;
• access control;
• information systems acquisition, development and maintenance;
• information security incident management;
• business continuity management;
• compliance.
The control objectives and controls in ISO 27001 are intended to be implemented to meet the requirements identified by a risk assessment. ISO 27001 is intended as a common basis and practical guideline for developing organizational security standards and effective security management practices, and to help build confidence in inter-organizational activities.
ISO/TS 16949
The ISO/TS 16949 is an ISO technical specification aiming to the development of a quality management system that provides for continual improvement, emphasising defect prevention and the reduction of variation and waste in the supply chain. It is based on the ISO 9001 and the first edition was published in March 2002 as ISO/TS 16949:2002. It was prepared by the International Automotive Task Force (IATF) and the "Technical Committee" of ISO. It harmonizes the country-specific regulations of Quality-Management-Systems.
About 30 percent of the more than 100 existing automobile manufacturers affiliate the requirements of the norm but especially the large Asian manufacturers have differentiated, own requirements for the quality management systems of their corporate group and their suppliers. TS 16949 applies to the design/development, production and, when relevant, installation and servicing of automotive -related products. It is based on ISO 9001.The requirements are intended to be applied throughout the supply chain. For the first time, vehicle assembly plants will be encouraged to seek ISO / TS 16949 certification.

The process-oriented approach to business processes that is addressed in the ISO 9001 stands at the forefront of the standard. It looks at the business processes in a process environment in which there are interactions and interfaces that need to be recognized, mapped and controlled by the quality management system. Additionally the gateways to the exterior (to sub-suppliers, customers and to remote locations) are defined. The Standard distinguishes between customer-oriented processes, supporting processes and management processes. This process-oriented approach is meant to improve the understanding of the whole process. Not an isolated process, but the entity of all interacting business processes affect the quality performance of a firm.
A key requirement of ISO / TS 16949 is the fulfillment of customer-specific requirements, set up by the automobile manufacturer in addition to the quality management system of their suppliers. This may have decisively contributed to the worldwide recognition of the TS by many manufacturers.
VDA 6.1 (Quality Management Standard of German Automotive Industry)
VDA 6.1 is a German quality standard similar to ISO/TS 16949, which is mandatory for part and component suppliers for the European automotive industry since 1999. The difference is that VDA 6.1 was established on the basis of ISO 9001 and ISO 9004-1.
The VDA (Verband der deutschen Automobilindustrie e.V.) - www.vda-qmc.de issued the 4th edition of the standard in 1998. In an effort to be consistent with the process orientation and wording of ISO 9001:2000, VDA made minor changes to this standard in 2003.
VDA 6.1 system requirements contain 2 main parts:
•Part M: Company Management (7 elements)
•Part P: Product and Process (16 elements)
The Part 'M' contains requirements such as management responsibilities, financial considerations, training, internal audits, corporate strategies, customer and employee satisfaction, as well as product liability and safety.
The Part 'P' contains requirements such as product & process related quality elements from a systematic perspective.
Within these two parts, in addition to the 20 elements of ISO 9001, VDA 6.1 composes the following special requirements:
05 Financial Considerations for Quality Management Systems
Costs for non-quality products, improvement of efficiency for processes, key performance indicators and financial reporting.
06 Recognition of Product Risk
Minimization of product risk during the production and assembly process, awareness of all employees about the impact of nonconformities on the product
Z1 Corporate Strategy
Regulates strategic business planning including measurement , analysis of business performance data as well as customer and employee satisfaction.
07 Contract review and quality in servicing
Focus on: 1) After Sales and 2) Market Feedback
For a better understanding, each question is explained by using practical examples followed by the suggested implementation method.
As far as the certification is concerned, all performance-related questions are evaluated quantitatively. The final result is expressed as a degree of conformity to the standard, using scale from 0 to 100%.
In order to pass the certification a company is expected to achieve at least 90%
Why Certification?
Certification by an accredited Certification Body certifies that your VDA 6.1 management system complies to accepted and internationally recognized standards and enhances the customer satisfaction. By using their extensive industrial expertise, the certification auditors shall assist to find improvement opportunities for the future development and to give your organisation the competitive edge.
In addition, certification provides advantages such as:
• Improved product and process quality
• Appropriate reassignment of limited resources
• Cost savings due to efficient operation restructuring
• Competitive advantages in global market
• Maximum exposure through media for the certification
• Co-ordinated quality assurance approach in the global supply chain
ISO / TS 29001
ISO/TS 29001 (published in 2003) defines the quality management system requirements for the design, development, production, installation and service of products for the petroleum, petrochemical and natural gas industries.
Developed as a direct result of a partnership between ISO and the international oil and gas industry (led by the American Petroleum Institute - API), ISO 29001 specifically focuses on the oil and gas supply chain.
The ISO / TS 29001 standard is based on ISO 9001 and incorporates supplementary requirements emphazing defect prevention and the reduction of variation and waste from service providers.
These requirements have been developed separately to ensure that they are clear and auditable. They also provide global consistency and improved assurance in the supply quality of goods and services from providers. This is particularly important when the failure of goods or services have severe ramifications for the companies and industries involved. This standard is for all organizations working within the oil and gas industry supply chain. Certification to ISO / TS 29001 ensures standardization and improvement within the sector.
Oil and gas industry players mainly manufacturers of oil industry equipment and materials, service providers (contractors, consultants etc.) and purchasers of equipment, materials and services can enhance their client’s satisfaction by applying this standard into their business model.
Benefits:

• A license to trade in the oil and gas industry. For many organizations within the oil and gas supply chain, certification to this standard is necessary to secure valuable contracts and gain competitive advantage.
• Enhanced brand reputation. Certification proves your commitment to industry best practice and enables you to stand out above the crowd.
• Flexibility. The standard has been designed to be compatible with other existing management systems standards making integrating your systems easier.
• Cost savings. Third-party certification to this technical standard will help to reduce multiple supplier audits and any associated costs.
• Managed business risk. Based as it is on ISO 9001, certification also makes it easier for you to measure performance and better manage business risk.
• Streamlined operations and reduced waste. The assessment focuses on your operating processes, which encourages you to improve the quality of your products and service and helps you to reduce waste, rejections and customer complaints.
• Encourages communication. Like ISO 9001, this requirement scheme ensures that employees feel more involved through improved communication. Continued assessment visits can highlight any problems and uncover any issues that may be present.
INTEGRATED MANAGEMENT SYSTEM (IMS) Certification
We can help you integrate your existing ISO 9001 system to ISO 14001, OHSAS 18001, ISO 22000 and achieve IMS certification.
The various integrated management system combinations are:
• ISO 9001, ISO 14001 & ISO 45001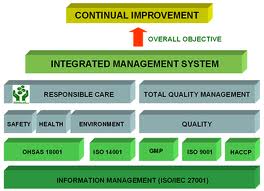

• ISO 9001, OHSAS 18001 & ISO 45001
• ISO 9001, ISO 14001, OHSAS 18001 & ISO 45001
• ISO 9001, ISO 14001, OHSAS 18001 & ISO 45001
• ISO 14001, OHSAS 18001 & ISO 45001
• ISO 9001, ISO 22000/HACCP
• ISO 9001, GMP/GHP
• HACCP, ISO 22000
For more information, please contact ZSR Vortices Office staff.










